As of September 20, 2021, a new fast-acting insulin called Lyumjev™ (pronounced LOOM-JEV) and manufactured by the company Eli Lilly has been approved by Health Canada.
At this time, this insulin is only approved for adults using a pen or syringe to inject their insulin. Research is currently underway to study its use in children and adolescents.
In the U.S., Lyumjev™ has been approved by the FDA for more than a year, and received approval last August for use in insulin pumps. In Canada, the application is currently pending.
Faster insulin for better control of post-meal blood glucose levels.
This ultra-rapid insulin contains the same active ingredient as Humalog® (Lispro), but has a faster-acting mode of action thanks to the addition of a molecule that activates the blood flow around the injection site. It should ideally be administered between 0 and 2 minutes before the start of the meal, but can be administered up to 20 minutes after the start of the meal, in rare cases where this might be required (e.g. forgetfulness, day of illness with risk of vomiting).
Studies show that Lyumjev™ provides better management of post-meal blood glucose levels (1h and 2h after the meal) when compared to Humalog®. It would therefore be particularly suitable for people who have difficulty controlling glucose levels after meals. This type of insulin could also show benefits for people using insulin pumps.
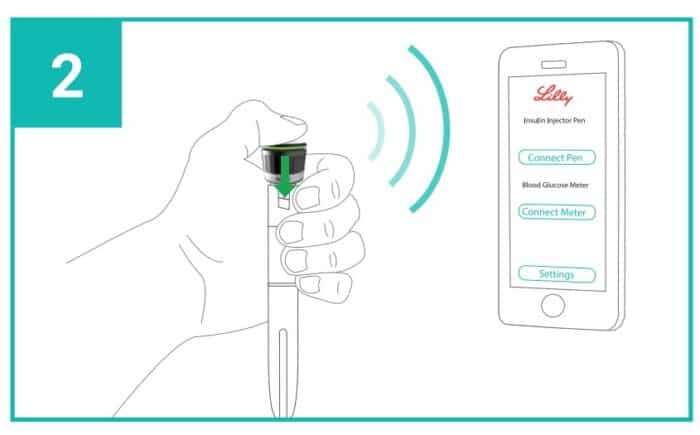
A new pen with the possibility of a Bluetooth connection.
In the United States, this insulin is available in a concentration of 100 and 200 units/ml, in the form of a vial, cartridge, pre-filled disposable pen, Junior Kwikpen (allowing adjustment to 0.5 units), but also in the form of a Tempo pen. This is a new disposable pen with a Bluetooth-enabled cap called Tempo Smart Button. This cap allows the pen to communicate with a smartphone and integrate the data directly into applications such as Dexcom or Glooko. No information is currently available regarding the approval of the Tempo Smart Button and its integration with Lyumjev™ insulin in Canada.

Availability and financial support
Despite Health Canada’s agreement, it remains to be seen when this insulin will be available to people living with diabetes. Indeed, Eli Lilly has decided not to market this insulin in the short and medium term in Canada.
It is therefore a matter to follow!

Did you know that we organize monthly webinars on topics related to type 1 diabetes?
Those webinar are presented in French but question could be asked and answered in English.
The next one will be held on September 28th and will focus on the financial aspects of type 1 diabetes. (RAMQ, private insurance, taxes, economic aid)
To participate (or to receive the registration) you just need to be registered in the Better Registry, a kind of census of people (adults and children) living with type 1 diabetes in Quebec.
Not yet registered? Do it here»
Participate in the BETTER registry!

First registry of people living with T1D in Canada.
Learn More